Ed - no intentional soaking but i might have spent too long at the torch fiddling around - and yes probably too hot all together makes sense. Thanks for taking time to reply.
Kevin - thanks for the in depth info. Where can i look up the correct temp to quench 1084?
You guys are great for helping me.
Kevin's web site has a great deal of amazing info on it including information for a number of steels. His site is my first place to check for any info when I am away from my library.
|quoted:
...Kevin - thanks for the in depth info. Where can i look up the correct temp to quench 1084?...
1084 is a great steel for anybody who wishes to work with more basic equipment for heat treating. The question is not so much a matter of whether soaking is detrimental to this steel, the real question is whether it is even necessary. Bladesmithing evolved and reached its zenith as an industry when the forge was the premier steel heating tool, at this time all steel was simply a mixture of iron and carbon; as simple as one can get. The material and the tools evolved to match each other and it worked. I have a saying that I repeat ad nauseam- “alloying changed everythingâ€. At the beginning of the 19th century (possibly earlier) the whole world changed as far as how we heat treat steel when steel makers started to intentionally add other elements to steel to alter its properties. It is no coincidence that we see whole new approaches to heating steel more accurately starting around this time as well. It is a simple rule- the more basic the alloy the more basic the tools required to heat treat it.
Simplicity in alloying comes from two directions- extra alloying elements and carbon content. Of the normally available steels the closest to the ancient iron-carbon steels from the age of the simple forge are the 10XX series steels, the primary extra alloying is just Mn. Within this family of steel now comes the carbon consideration, those with less than .8% carbon have special considerations due to the iron to carbon ratio, and those with more than .8% carbon have special considerations due to the iron to carbon ratio. Steel falling right in the .8% carbon range take the least effort and concern to heat treat do to an ideal iron-carbon ratio. 1084 is so close to that ideal .8% (known as the eutectoid) that it goes into solution, and is ready to quench, very readily.
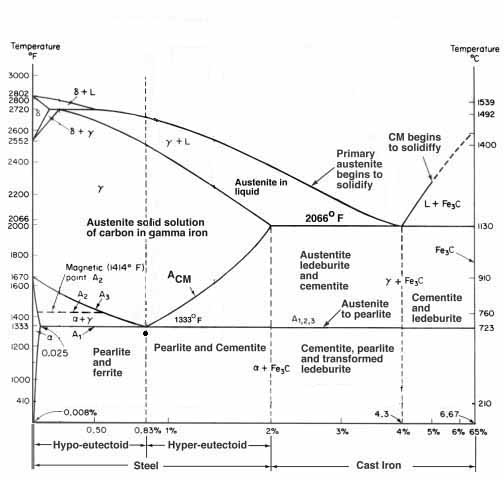
Here is an iron-carbon equilibrium diagram that shows the phases we get from heating different carbon contents to a given temperature, if our steel were just iron and carbon, which it is not but this is as close as it gets. Here the eutectoid is shown as .83% (close enough, but great for our consideration of 1084). You will notice that in a basic iron-carbon alloy the transformation to the solution that we need to quench from begins at 1333F (line A1 in the diagram), but to the left another line climbs at an angle away from the eutectoid at .83%C and 1333F. This line (A3) tells you how much more temperature that is required to saturate the iron with the limited carbon levels below .83%. To the right there is another line climbing on an angle (ACm) that shows how much more temperature is needed to put increasing amounts of carbide into solution.
At first glance it would appear that 1333F would be all you need to quench 1084, however…
This is an equilibrium diagram, which means it assumes time has already been accounted for and so does not consider it. Also, we must always remember that we are not just dealing with iron and carbon, every bar of steel has something else in there; for 1084 it is a healthy dose of Mn and any number of trace elements. Because of this standard practices recommend 1500F for 1084 but for our purposes a very user friendly range from 1450F to 1500F is available to us.
Now, if undissolved constituents keep the grain boundaries stable and a .83% steel is in total solution at 1333F, wouldn’t that result in grain growth as soon as you reach or exceed 1333F? No, because of that whole equilibrium thing again. I have the benefit of actually working with the simple iron carbon steels that our ancestors used when I make my own bloomery steel, and I can tell you that we have very little to worry about with the grain of modern steel compared to that stuff.
Those old steels were similar to what the steel industry would call a coarse grained steel, this does not mean that they had course grain, but instead referred to steels that industry used silicon based methods to kill when casting the ingot. When steel is poured into the ingot form it produces a lot of gas, this gas will fill the solidified product with porosity not too different from Swiss cheese. Obviously this is bad if you want a good solid bar of steel, so early on industry found that adding silicon would “kill†this gassing problem. This steel, like its predecessors would commence with grain growth almost as soon as full solution was reached in heat treatment and thus would often have enlarged grain if given any time at temperature, so it was labeled course grained steel.
But then, actually some time ago, industry found that aluminum was much more effective in killing the steel when poured, but something really interesting also happened, the process formed aluminum nitride particles that help stabilize the grain boundaries. Since this steel showed no grain growth, where the coarse grained steel did, it was called “fine grained†steel. But here is the difference- while course grained steel began steady grain growth as soon as carbon solution was achieved, the fine grained steel had particles that resisted going into solution and thus this steel would maintain its grain size until it reached the temperature at which the extra particles would dissolve, at which point the grains would grow very rapidly. This give you an idea of how any of these extra bits and pieces in our modern steels change the game as far as solution and grain growth. The V in W2 tool steel is there almost exclusively as a grain refiner (V carbide takes a whole lot of heat to dissolve).
Since 1084 goes into solution so well in that very friendly and wide range one can get very nice results with temperature alone and often just dispense with soaking. But moving on to its big brother 1095 you run into a problem. Every % of carbon over that eutectoid range will tend to be too much in solution and will give you problems (that would be another monster post or thread to describe). So the much safer way to do it is to nibble away at the iron carbide to strip off just the right amount of carbon in solution, so you lower the temperature and allow time to do the work more effectively via the soak. You can eliminate time just by throwing more temperature at it but that would be like adjusting a crooked painting on the wall with a several ton wrecking ball.
…I have more information for you that I will put in another post, but I just got told that I need to help my daughter study for a test… a test on physics, of all things! <img src=' http://www.americanbladesmith.com/ipboard/public/style_emoticons//biggrin.gi f' class='bbc_emoticon' alt=':D' />
"One test is worth 1000 'expert' opinions" Riehle Testing Machines Co.
Wow - thanks Kevin, that is an amazing amount of helpful information. I really appreciate the help.
O.K. now that my daughter has given me a refresher course in 9th grade Newtonian physics we can get back at it here.
Here is a chart I made for a lecture I did on advanced heat treating concepts, it is based on a series of tests I did with common blade steels to determine the effects of soak time on as-quenched hardness.

What will immediately become apparent is how the simpler steels have a much lesser difference in hardness than those with more alloying, with 1084 being around a half point Rockwell. This illustrates what I previously said about whether soaking is even necessary with simpler steels.

Here is a chart from the same lecture showing the differences in HRC readings on the same piece of un-soaked material. You see, I never take just one HRC reading, but rather a series of 5 or more and then average the results for my final number to compensate for deviations in the numbers. The ideal is to have less than 1 point deviation in the series, indicating that the internal hardened condition of the steel is homogenous via even carbon solution (austenite). You will notice here that the un-soaked deviation is a clear reflection of the amount of alloying present. The more alloying (including carbon), the more beneficial the soak.
This is what is going on here:
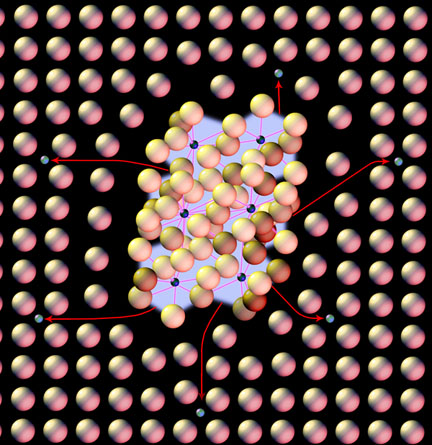
In this illustration you have the matrix of repeating iron atoms within the steel, when not in solution the carbon (the little darker atoms) bonds up into iron-carbide particles. To harden the steel you need that carbon scattered between the iron atoms in the matrix, so those carbon atoms have to first be broken free of that bonded carbide and then dispersed into the matrix. The heat breaks the bonds but you still need time to diffuse the carbon evenly. Iron carbide dissolves pretty easily but alloying, such as chromium, forms a much stronger bond with the carbon and thus needs more time to break free and move about. Many of these alloys also tend to make larger carbides with greater distances between them in the matrix, complicating things even more. If you simply dissolve the carbide without allowing time for even diffusion in the matrix you will get deviations in hardness due to uneven carbon levels throughout.
Here is an image of 5160 that had no soak time whatsoever, it was taken exactly to non-magnetic and immediately quenched:

Here you can actually see the high and low carbon concentrations where the carbide phases are half dissolved, leaving patches of ferrite (iron), and weak martensite. This sample measured 47HRC but would strip the teeth off a file, illustrating the shortcomings of some methods over others in determining complete hardening (files don't measure penetrative hardness like Rc testers do).

Finally we have an image showing O-1 with increasing soak times. In these the lighter white/bluish particles are carbide, the tannish brown colored background is the martensite we want for a hardened blade. In the first image 2 minutes of soak time gave us some martensite but vast amounts of leftover carbide, this piece would show a lower Rockwell. The next image is of O-1 soaked for 10 minutes and you can see that we still have the larger carbides but the haze, caused by the finer carbides, has lifted to reveal much more martensite, this piece would have a good hardness and those extra carbides would add wear resistance to our edge. The final image is a piece of 0-1 soaked for 5 hours (not a typo I actually mean HOURS). Here you see only the largest carbides (now much smaller) still remain and the entire sample is carbon saturated martensite. While this may seem good, all that carbon in solution created a more brittle martensite loaded up with tons of retained austenite, so it is both too brittle and too soft, at the same time, for a stable edge. However directly below the image is the fractured end of that piece to show you that the 5 hours had no effect at all on grain size because the temperature was constant and controlled. But this is also an excellent example of how a great blade is an entire package that is not achieved by focusing on just one thing, in this case- great grain size, but still a lousy knife.
"One test is worth 1000 'expert' opinions" Riehle Testing Machines Co.
Now here is some good news. I am going to give a bit of a preview now of material that I have been working on writing on how we can prepare steels to behave more like their simpler ancestors and be more friendly with basic heat sources such as a forge. As we have already discussed, the courser and more segregated the carbide particles in the steel, the more effort it takes to homogenize the steel for hardening. Most high carbon steel comes to us in a spheroidized annealed condition, this makes machining very easy but also requires greater effort in temperature and time to put things back into solution.
The problem, and solution, is actually straight forward when you look at it. Annealing is the heat treatment we do to soften the steel, hardening is the opposite. Going straight from the annealed condition into hardening puts the two extremes at odds with each other. If we want to reduce or eliminate the need for things that forges are not good at, like soak times, we need to get the steel ready to go into solution more easily. For this we want to make our carbides much finer and more numerous in an evenly scattered pattern. Then, when we heat the steel to harden it the finer carbides will quickly dissolve and the carbon will have much less distance to travel for homogenization.
Simple normalizing is about the best thing you can do to set this up for success. If you do the old trick with wood ash or vermiculate to anneal your steel you will probably need some degree of soaking to get proper solution, and with something like 1095 it can be so detrimental that even a good soak won’t help. Whether it is spheroidized or full annealed in wood ash or vermiculite, the carbide is probably segregated enough to need a soak. But if you put it all back into solution and then just air cool, instead of the slower methods, you will form finer structures that will not require the soak, and the simpler the steel the more this will be the case. However be aware that this comes from dissolving the coarse structures to begin with, excessive low temperature cycling increases the segregation and sets you back a bit.
"One test is worth 1000 'expert' opinions" Riehle Testing Machines Co.
Oh, and by the way- Jim, normalize your 1084 blade and then heat from 1475F to 1500F, quench immediately, and you should get a nice 65HRC... that was the quick answer <img src=' http://www.americanbladesmith.com/ipboard/public/style_emoticons//biggrin.gi f' class='bbc_emoticon' alt=':D' />
"One test is worth 1000 'expert' opinions" Riehle Testing Machines Co.
Amazing Kevin - thanks for such a great in-depth answer.
-Jim
|quoted:
Oh, and by the way- Jim, normalize your 1084 blade and then heat from 1475F to 1500F, quench immediately, and you should get a nice 65HRC... that was the quick answer <img src=' http://www.americanbladesmith.com/ipboard/public/style_emoticons//biggrin.gi f' class='bbc_emoticon' alt=':D' />
<img src=' http://www.americanbladesmith.com/ipboard/public/style_emoticons//laugh.gi f' class='bbc_emoticon' alt=':lol:' />

